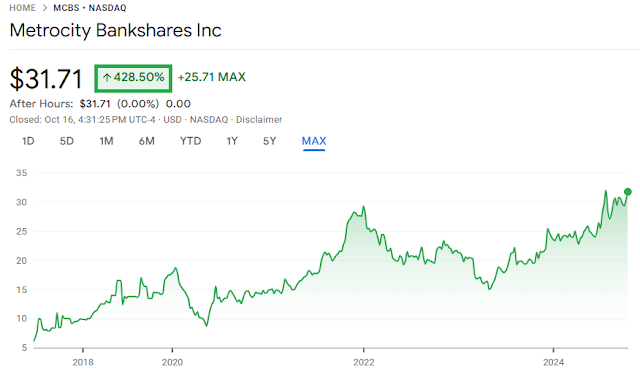
MetroCity Bankshares increases quarterly cash dividend to $0.23/share from $0.20/share and announces the continuation of its share repurchase program
- The Company approves the continuation of its share repurchase program that expired on September 30, 2024 and authorized the Company to repurchase up to 925,250 shares of the Company's outstanding shares of common stock, which is the number of remaining shares authorized for repurchase from the Prior Share Repurchase Plan.
- The share repurchase program will begin on October 17, 2024 and end on September 30, 2025.
| Earnings Date | Oct 18, 2024 |
| Forward Dividend & Yield | 0.80 (2.58%) |
| Ex-Dividend Date | Jul 31, 2024 |
Dividend Payable Date Aug 9, 2024