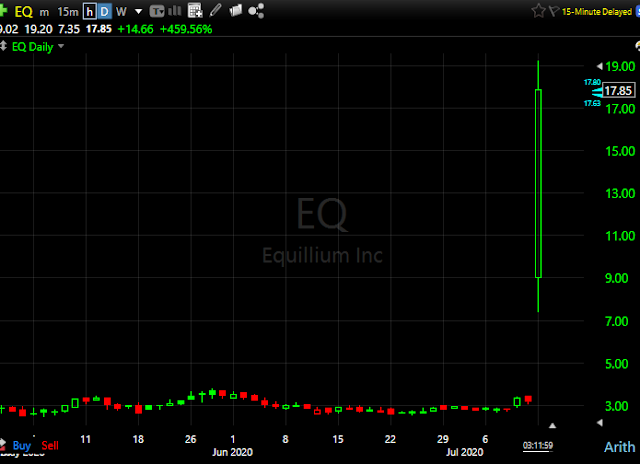
Equillium (EQ) announced that a clinical trial conducted in India by its partner Biocon demonstrated that itolizumab significantly reduced mortality in patients hospitalized with COVID-19.
Equillium reports data for Itolizumab demonstration reduction in mortality in patients hospitalized with COVID-19
Co announced that as reported by its partner, Biocon Limited, a clinical trial conducted in India by Biocon demonstrated that itolizumab significantly reduced mortality in patients hospitalized with COVID-19. Biocon has announced that the Drugs Controller General of India (DCGI), the regulatory agency that oversees drug approvals, has granted restricted emergency use of itolizumab for the treatment of cytokine release syndrome (CRS) in COVID-19 patients with moderate to severe acute respiratory distress syndrome (ARDS) in India. Based on the encouraging topline results of the study reported by Biocon and subsequent DCGI approval to treat COVID-19 patients, Equillium is planning to conduct a global randomized controlled clinical trial of itolizumab in COVID-19 patients for which it will file a U.S. investigational new drug application (IND).
Biocon conducted a randomized, controlled, open-label study at four hospitals in India, enrolling a total of 30 hospitalized COVID-19 patients with moderate to severe ARDS. Twenty patients were randomized to receive itolizumab plus best supportive care, while 10 patients received best supportive care alone. The primary endpoint was mortality at one month. As reported by Biocon:
- In the itolizumab arm there were no deaths and all patients have recovered; in the control arm three patients died and the remainder have recovered.
- The mortality benefit observed in the itolizumab arm was statistically significant.
- Consistent with the observed clinical improvement, patients who received itolizumab also experienced significant reductions in inflammatory cytokines such as IL-6 and TNFa.





No comments:
Post a Comment